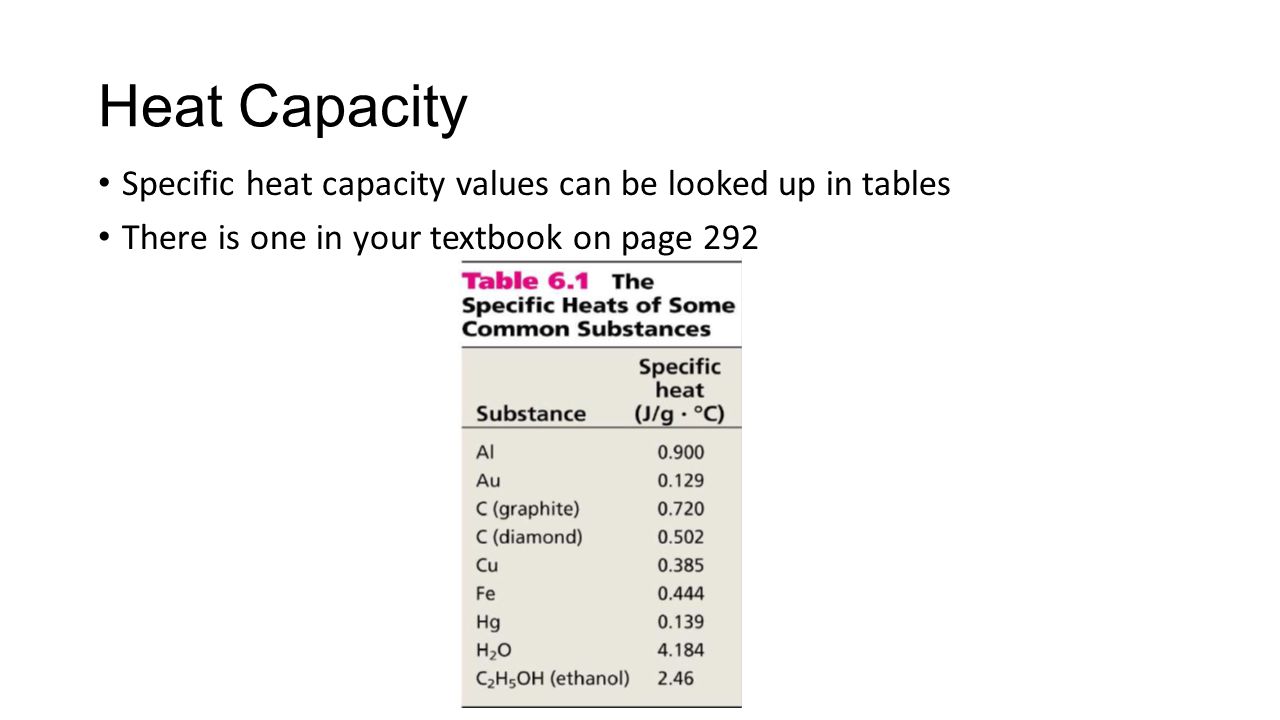
Equal volumes, 50.0 mL, of 3.0 M hydrochloric acid and 3.0 M sodium hydroxide solutions having an initial temperature of 20.0°C react in a calorimeter. The resultant solution records a temperature of 40.0°C. The heat gained by the resultant solution can be calculated using. Sodium hydroxide, also known as lye. Heat capacity (C) 59.5 J/mol K Std molar entropy (S o 298). Specific foods processed with sodium hydroxide include. Go To: Top, Solid Phase Heat Capacity (Shomate Equation), References Data from NIST Standard Reference Database 69: NIST Chemistry WebBook The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of. The DOW reference is good because they make Sodium Hydroxide. A good library reference on chemicals is Perry's Handbook of Chemical Engineering. It has a table of Sodium Hydroxide heat capacities for solutions. And NO, the density of a solution is NOT the same as water unless it is very dilute. Swampie777 ( Chemical Engineer).
- Formula: HNaO
- Molecular weight: 39.9971
- IUPAC Standard InChI:
- InChI=1S/Na.H2O/h;1H2/q+1;/p-1
- Download the identifier in a file.
- IUPAC Standard InChIKey:HEMHJVSKTPXQMS-UHFFFAOYSA-M
- CAS Registry Number: 1310-73-2
- Chemical structure:
This structure is also available as a 2d Mol fileor as a computed3d SD file
The 3d structure may be viewed usingJavaorJavascript. - Species with the same structure:
- Information on this page:
- Other data available:
- Data at other public NIST sites:
- Options:
Data at NIST subscription sites:
NIST subscription sites provide data under theNIST Standard ReferenceData Program, but require an annual fee to access.The purpose of the fee is to recover costs associatedwith the development of data collections included insuch sites. Your institution may already be a subscriber.Follow the links above to find out more about the datain these sites and their terms of usage.
Solid Phase Heat Capacity (Shomate Equation)
Go To:Top, References, Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Cp° = A + B*t + C*t2 + D*t3 + E/t2
H° − H°298.15= A*t + B*t2/2 + C*t3/3 + D*t4/4 − E/t + F − H
S° = A*ln(t) + B*t + C*t2/2 + D*t3/3 − E/(2*t2) + G
Cp = heat capacity (J/mol*K)
H° = standard enthalpy (kJ/mol)
S° = standard entropy (J/mol*K)
t = temperature (K) / 1000.
View plotRequires a JavaScript / HTML 5 canvas capable browser.
| Temperature (K) | 298. - 572. | 572. - 596. |
|---|---|---|
| A | 419.4837 | 86.02304 |
| B | -1717.754 | 0.000000 |
| C | 2953.573 | 0.000000 |
| D | -1597.221 | 0.000000 |
| E | -6.046884 | 0.000000 |
| F | -517.8662 | -448.8512 |
| G | 933.0738 | 169.6281 |
| H | -425.9312 | -425.9312 |
| Reference | Chase, 1998 | Chase, 1998 |
| Comment | Data last reviewed in December, 1970 | Data last reviewed in December, 1970 |
| Temperature (K) | Cp (J/mol*K) | S° (J/mol*K) | -(G° - H°298.15)/T (J/mol*K) | H° - H°298.15 (kJ/mol) |
|---|---|---|---|---|
| 298. | 59.52 | 64.43 | 64.44 | -0.00 |
| 300. | 59.67 | 64.83 | 64.45 | 0.12 |
| 400. | 64.94 | 82.71 | 66.85 | 6.34 |
| 500. | 75.16 | 98.17 | 71.59 | 13.29 |
| Temperature (K) | Cp (J/mol*K) | S° (J/mol*K) | -(G° - H°298.15)/T (J/mol*K) | H° - H°298.15 (kJ/mol) |
|---|---|---|---|---|
| 572. | 86.02 | 121.6 | 75.62 | 26.29 |
References
Go To:Top, Solid Phase Heat Capacity (Shomate Equation), Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Specific Heat Capacity Formula
Chase, 1998
Chase, M.W., Jr.,NIST-JANAF Themochemical Tables, Fourth Edition,J. Phys. Chem. Ref. Data, Monograph 9, 1998, 1-1951. [all data]

Notes
Go To:Top, Solid Phase Heat Capacity (Shomate Equation), References
Specific Heat Capacity Of Sodium Hydroxide
- Data from NIST Standard Reference Database 69:NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST)uses its best efforts to deliver a high quality copy of theDatabase and to verify that the data contained therein havebeen selected on the basis of sound scientific judgment.However, NIST makes no warranties to that effect, and NISTshall not be liable for any damage that may result fromerrors or omissions in the Database.
- Customer supportfor NIST Standard Reference Data products.